Priority Review! Hansoh Pharma Applies for Marketing Approval of First-Line Treatment Indication for Ameile
Recently, the marketing approval application of Ameile (Almonertinib Mesilate Tablets), Class 1 innovative drug independently developed by Jiangsu Hansoh Pharmaceutical Group Co., Ltd. ("Hansoh Pharma") for first-line treatment of EGFR mutation-positive locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation in adult patients, has been accepted by the National Medical Products Administration, and has been included in the Publicity List of Varieties Subject to Priority Review by the Center for Drug Evaluation of National Medical Products Administration. This is the second indication for Ameile.
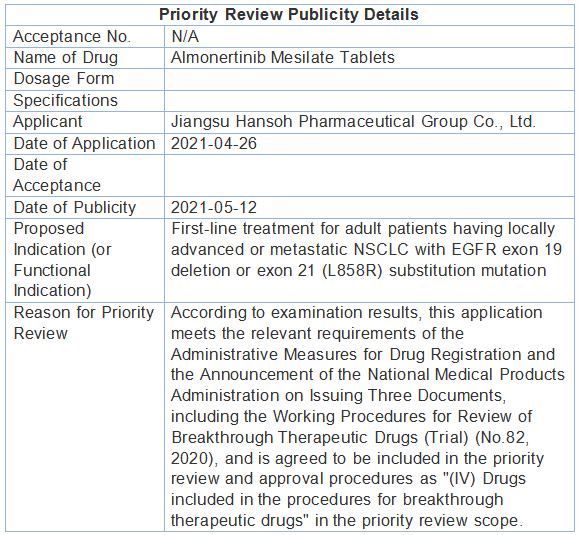
▲ Screenshot of the publicity details on the official website of the National Medical Products Administration After the approval for marketing of the first indication, Hansoh Pharma has further initiated a number of clinical studies to explore the therapeutic potential of Ameile in the lung cancer segment. For the indication under application for marketing this time, the Phase III study on first-line treatment of EGFR mutation-positive locally advanced or metastatic NSCLC has reached the primary study endpoint, and the latest study results will be released at ASCO 2021, which is worth looking forward to. Ameile is a novel, irreversible epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) with promising pharmacological properties that selectively inhibits EGFR sensitive and tolerant mutations. It is China's first original third-generation EGFR-TKI innovative drug, approved for marketing in March 2020 to treat NSCLC and address the drug resistance problem of T790M; and also the world's first third-generation EGFR-TKI with median progression-free survival (mPFS) exceeding one year (second-line use), included in the National Reimbursement Drug List in December 2020, providing a new clinical option that is potent, safe and accessible, and bringing hope for long-term, high-quality survival to more patients with advanced NSCLC.


